Introduction
Exponential growth in solar and wind renewable energy generation has the potential to create grid instability. Both solar- and wind-generated energy are intermittent sources that fluctuate in energy delivered to the grid. Without substantial increases in grid-scale energy storage, it will be difficult for solar and wind-generated energy to become greater than 30-40 percent of our total energy generation. Fortunately, technology advances in large-scale batteries, fuel cells, and electrolyzers have been made recently.
This article describes a broad overview of some shorter term, mid-term, and longer-term options for energy storage that takes into account variable factors.
Reduced solar energy production output is experienced at night, during high cloud cover and inclement weather. New technologies, which store photovoltaic energy for generation shortfall periods will enable renewable energy to account for a higher percentage of total electricity production and reduce the base load requirements of fossil fuel-generated electricity production. Figure 1 shows the daily electricity demand in California (2013 CAISO report). Daily solar energy production peaks at noon and tapers off at 3 to 4 p.m. creating shortfalls from 5 to 9 p.m. and from 6 to 8 a.m.
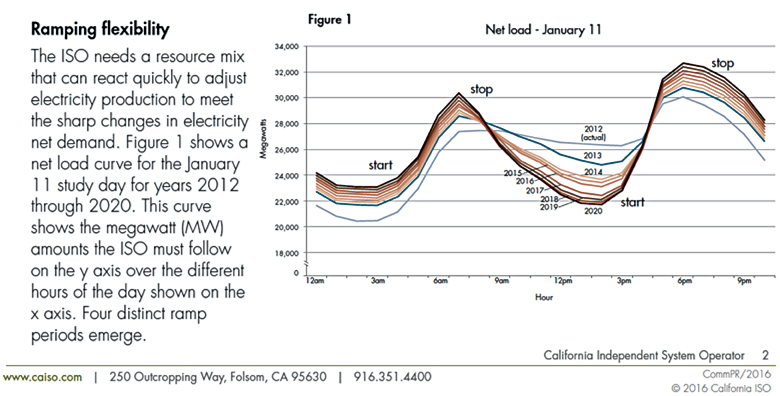
Figure 1
(click to enlarge)
Lithium-ion Batteries-Shorter Term
Increased investment in Lithium-ion battery storage can bridge the hourly/daily shortfalls between solar energy production and net
electricity demand. Over the past five years, lithium-ion batteries [1] have dominated electrochemical grid storage. This dominance is due to the effort over the last two decades in research and manufacturing for energy storage in portable electronic devices. This effort, combined with the growing installed cell manufacturing capacity, has resulted in storage costs going from $1500/kWh in 2006 to $300/kWh in 2014 [2]. A number of companies are offering lithium-ion battery energy storage systems that are cost competitive with diesel generators and lead acid batteries in grid storage applications [3, 4]. Typical ideal applications require less than four hours of operation or have rapid charge/discharge cycles, frequency regulation.
Lithium-ion battery describes the combination of different anode and cathode materials. The most commonly utilized anode material – graphite – was first commercially paired with a lithium cobalt oxide cathode to power early-generation camcorders and laptops [5]. A shift to higher-energy density and greater safety needs for large-scale operation led to the adoption of new chemistries. A partial replacement of graphite with silicon, such as silicon alloy [6], and replacement of the lithium cobalt oxide with lithium nickel manganese cobalt oxide have resulted in cells with more energy while improving safety.
Battery system architecture should be designed to allow sufficient, uniform, temperature regulation. Failure to address either of these can result in premature capacity loss, system failure and cell thermal runaway. Interest in this area has become great enough that industry-sponsored committees are developing standards around this topic [7] [8].
The future of lithium-ion batteries in grid-scale energy storage is exciting. As companies continue to scale up manufacturing capabilities, and raw material suppliers improve their yields, the market will see costs come down. Researchers will improve the energy density, life and safety through material advancements. Two areas of particular interest: development of solid electrolyte systems to replace flammable electrolytes [9] and high-voltage stable electrode materials to enable longer operational life [10].
Flow Batteries-Medium Term
Medium term renewable energy-generated electricity shortfalls include days of low wind speed, cloud cover, and nighttime electricity demand lasting more than four hours. One energy storage system experiencing rapid growth to meet the challenge is a redox flow battery (RFB). Under development for over three decades, it is touted for specifying system power independent from operational run time [11]. A long-running commercial demonstration is being conducted by Sumitomo Electric Industries with a calendar lifetime of over a decade [12]. Newcomers such as Vionx, Rongke Power and ViZn have recently entered the market [13] [14] [15].
The RFB is as much a chemical plant as it is a battery. Its design utilizes holding tanks containing active species dissolved in an electrolyte [11]. During charge and discharge, two mixtures--one cathode side and one anode side--are pumped through electrode plates called a stack. Within the stack is a repeating series of graphite-based bipolar plates and porous carbon electrodes separated by an ionically selective permeable membrane. As the mixtures pass through the porous electrodes, electrons are added or removed from the active redox species with the corresponding counter-ion passing through the membrane. The fluid is returned to the original holding tank, where it can be stored for future use or pumped through for further oxidation and reduction. Similar to a chemical reactor an important figure of merit is first-pass conversion efficiency.
To gain greater market adoption, manufacturers are improving the maturity and cost. The three biggest cost contributors to the system are the electrolyte, membrane and the porous carbon electrode. The electrolytes most widely used are aqueous based containing expensive metals like vanadium [16]. To reduce costs, efforts are being explored to use iron and zinc, or replace the metals with organic molecules [17] [18]. Due to the low chemical crossover requirements, expensive fluoropolymer membranes are often used [19]. To reduce costs and improve performance, designers are exploring thinner constructions or switching to hydrocarbon-based materials. Reducing system costs coupled with improved understanding of degradation mechanisms and operation life can accelerate adoption.
Electrolyzers-Longer Term
Longer-term renewable energy storage (>24 hours) will require that renewable energy be stored as a fuel in geographies not suitable for hydroelectric storage. Hydrogen can be generated using an electrolyzer and excess renewable energy. The process utilizes electricity to split water molecules into its chemical constituents, hydrogen and oxygen. Combining the fuel generation of an electrolyzer with energy production of a fuel cell creates a system where energy can be stored for extended periods. Such a system also provides independence of power and energy sizing with the use of aggressive chemicals.
PEM water electrolyzers operate at mild operating conditions with temperatures generally at or below 80°C and use perfluorosulfonic acid-based membrane as an ionic (protonic) conductor. Construction utilizes a membrane in direct contact with a Pt-based H2 generating catalyst on the cathode and Ir-based O2 generating anode for a catalyst coated membrane (CCM). The CCM is sandwiched between plates containing an arrangement of channels for evacuation of produced gases and ensuring a reliable water supply. When a voltage in excess of 1.23V is applied generation of H2 and O2 from water begins. The reaction rate depends on factors, including the key parameters of applied over potential, temperature, pressure, catalyst activity and active area, and membrane conductivity.
Progress in PEM electrolyzer development has grown in recently following decades of stagnation. Historical markets for generated hydrogen that are served by PEM electrolyzers include wafer cleaning, turbine cooling and lab grade instrumentation. These markets value the gas purity and reliability of supply offered by PEM electrolyzers. Cost (both operational and capital) was of relatively secondary importance -- there was no pressure to improve. The situation has changed in recent years with the substantial growth in renewable energy generation causing destabilization of power grids.
Fuel Cells-Generating Electricity from Hydrogen
Fuel cells are electrochemical devices that convert chemical energy into electrical energy. They differ from batteries in one key aspect: rather than having the chemical reactants sealed in a single container, they are open systems where fuel and oxidant can continuously feed into the device, and the byproduct of the reaction removed. This allows the cell to generate electricity as long as the reactants are supplied. While this may be possible for many electrochemical reactions, only those that produce benign byproducts such as water and carbon dioxide are of interest for venting into the atmosphere. Fuel cells that operate on hydrogen and ambient supplied oxygen are of interest since they can be highly efficient and produce water as the only byproduct.
There are several types of fuel cells that are emerging for commercial applications often classified by the type of electrolyte used in the cell. Proton exchange membrane fuel cells (PEMFCs) operate at much lower temperatures (<100°C) where the electrolyte is a polymer-based membrane. This system is suited for back-up power or transportation since it can quickly start-up, shut-down, and respond to electrical load demands.
One downside to converting electricity into hydrogen and back is the roundtrip efficiency of the process. Electrolyzers are about 80 percent efficient; fuel cells are about 60 percent efficient. This means a little less than half of the original electricity (48 percent) is returned at the cycle end.
Conclusion
The world of energy storage is not a one-size-fits-all. One must consider many factors including operational run time, system footprint and total system costs. For applications needing less than half a day operation, systems like lithium ion or flow batteries, may be the more cost effective choice. When longer storage times are needed, converting electricity to hydrogen allows the energy to be stored for periods of time that would be economically impractical for traditional batteries. A diversity of renewable energy storage technologies will enable the grid to use higher percentages of renewable energy, possibly even 100 percent renewable energy, as shown in Figure 2.
Figure 2
(click to enlarge)
About the Authors
 Brandon Bartling, advanced product development specialist in the 3M Energy & Electronics Business Group Lab, graduated from Case Western Reserve University with a Ph.D. in chemical engineering, and has 14 years of energy storage and electrochemistry experience. Bartling spent the beginning of his career working for Energizer Battery where he focused on research and engineering efforts in the areas of Zn-MnO2, Zinc-Air and Zinc-Silver Oxide cells. After finishing his Ph.D., Bartling worked at General Electric Global Research facility for four years developing a high temperature sodium-nickel battery chemistry. He now conducts research at 3M in the area of energy storage for a variety of chemistries including redox flow batteries, lithium-ion batteries and other energy storage systems
Brandon Bartling, advanced product development specialist in the 3M Energy & Electronics Business Group Lab, graduated from Case Western Reserve University with a Ph.D. in chemical engineering, and has 14 years of energy storage and electrochemistry experience. Bartling spent the beginning of his career working for Energizer Battery where he focused on research and engineering efforts in the areas of Zn-MnO2, Zinc-Air and Zinc-Silver Oxide cells. After finishing his Ph.D., Bartling worked at General Electric Global Research facility for four years developing a high temperature sodium-nickel battery chemistry. He now conducts research at 3M in the area of energy storage for a variety of chemistries including redox flow batteries, lithium-ion batteries and other energy storage systems
 Tim Hebrink, staff scientist in 3M Corporate Research Lab, graduated from the University of Minnesota with a BS in chemical engineering, and has 32 years of polymer product development experience at 3M. His expertise and innovations in polymer properties and polymer processing has earned him 51 issued patents and 66 pending patent applications covering novel optical polymers, optical film designs, polymer films with improved properties, and novel applications of polymer films. Polymer films produced by his inventions have enabled significant 3M polymeric film sales resulting in 6 Golden Step Awards for individual products achieving sales greater than $15million/year and cumulative sales of greater than $5billion. He has held several positions ranging from polymer synthesis and polymer processing to environmental engineering and polymer film application development. He has also co-authored 14 research publications including a book chapter on Durable Polymer Films.
Tim Hebrink, staff scientist in 3M Corporate Research Lab, graduated from the University of Minnesota with a BS in chemical engineering, and has 32 years of polymer product development experience at 3M. His expertise and innovations in polymer properties and polymer processing has earned him 51 issued patents and 66 pending patent applications covering novel optical polymers, optical film designs, polymer films with improved properties, and novel applications of polymer films. Polymer films produced by his inventions have enabled significant 3M polymeric film sales resulting in 6 Golden Step Awards for individual products achieving sales greater than $15million/year and cumulative sales of greater than $5billion. He has held several positions ranging from polymer synthesis and polymer processing to environmental engineering and polymer film application development. He has also co-authored 14 research publications including a book chapter on Durable Polymer Films.
 Michael Yandrasits is currently the Electrochemical Components Lab membrane research group leader in 3M’s Corporate Materials Research Lab. He earned his bachelor’s degree in chemistry from Illinois State University and Ph.D. in Polymer Science from the University of Akron. Yandrasits has worked in 3M’s research labs for 35 years in, the last 17 years in fuel cell membrane development. He has more than 30 issued US patents primarily in the area of fuel cell technology. He has been the principle investigator for two Department of Energy-funded contracts in the proton exchange membrane field and most recently an Advanced Research Projects Agency- Energy (ARPA-E) grant to develop commercially viable anion exchange membranes.
Michael Yandrasits is currently the Electrochemical Components Lab membrane research group leader in 3M’s Corporate Materials Research Lab. He earned his bachelor’s degree in chemistry from Illinois State University and Ph.D. in Polymer Science from the University of Akron. Yandrasits has worked in 3M’s research labs for 35 years in, the last 17 years in fuel cell membrane development. He has more than 30 issued US patents primarily in the area of fuel cell technology. He has been the principle investigator for two Department of Energy-funded contracts in the proton exchange membrane field and most recently an Advanced Research Projects Agency- Energy (ARPA-E) grant to develop commercially viable anion exchange membranes.
 Krzysztof Lewinski is a research specialist for the Electrochemical Components Lab in 3M’s Corporate Materials Research Lab.
Krzysztof Lewinski is a research specialist for the Electrochemical Components Lab in 3M’s Corporate Materials Research Lab.
References
[1] Sandia National Laboratory, 2017. [Online]. Available: www.energystorageexchange.org.
[2] B. Nykvist and M. Nilsson, “Rapidly falling costs of battery packs for electric vehicles,” Nature Climate Change, pp. 329-332, 2015.
[3] “Powerwall,” Tesla, 2017. [Online]. Available: www.tesla.com/powerwall.
[4] NEC Energy Solutions, “NEC Energy Solutions,” NEC, 2017. [Online]. Available: www.neces.com.
[5] Sony, “Sony History,” Sony, 2017. [Online]. Available: www.sony.net/SonyInfo/CorporateInfo/History/SonyHistory/2-13.html.
[6] R. Turner, D. McClure, L. Krause, M. Buckett, J. Dahn and O. Mao, “Electrode for a lithium battery”. United States Patent 6203944B1, March 1998.
[7] IEEE, “IEEE PES Energy Storage and Stationary Battery Committee,” IEEE, 2017. [Online]. Available: sites.ieee.org/pes-essb.
[8] National Fire Protection Association, “Codes & Standards,” National Fire Protection Association, 2017. [Online]. Available: www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code-855&tab=committee.
[9] E. Rangasamy, J. Wolfenstine and J. Sakamoto, “The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2012,” Solid State Ionics, vol. 206, no.5, pp. 28-32, 2012.
[10] K. Kang, Y. Meng, J. Breger, C. Grey and G. Ceder, “Electrodes with High Power and High Capacity for Rechargeable Lithium Batteries,” Science, vol. 311, no. 5763, pp. 977-980, 2006.
[11] A. Z. Weber, M. M. Mench, J. P. Meyers, P. N. Ross, J. T. Gostick and Q. Liu, “Redox flow batteries: a review,” Journal of Applied Electrochemistry, vol. 41, p. 1137, 2011.
[12] T. Shibata, T. Kumamoto, Y. Nagaoka and K. Kawase, “Redoc Flow Batteries for the Stable Supply of Renewable Energy,” Sumitomo Electric Industries, 2003.
[13] Vionx Energy, 2017. [Online]. Available: vionxenergy.com
[14] “Rongke Power,” [Online]. Available: www.rongkepower.com
[15] ViZn Energy, [Online]. Available: www.viznenergy.com.
[16] M. Skyllas-Kazacos, G. Kazacos, G. Poon and H. Verseema, “Recent advances with UNSW vanadium-based redox flow batteries,” International Journal of Energy Research, vol. 34, no. 2, pp. 182-189, 2010.
[17] B. Huskinson, M. P. Marshak, C. Suh and M. J. Aziz, “A metal-free organic-inorganic aqueous flow battery,” Nature, vol. 505, no. 7482, pp. 195-198, 2014.
[18] K. L. Hawthorne, J. S. Wainright and R. F. Savinell, “Studies of Iron-Ligand Complexes for an All-Iron Flow Battery Application,” Journal of the Electrochemical Society, vol. 161, no. 10, pp. A1662-A1671, 2014.
[19] S. J. Hamrock, L. M. Rivard, G. Moore and H. T. Freemyer, “Polymer electrolyte membrane”. US Patent US7348088, 25 March 2008.







